How To Find Electronegativity With Periodic Table
Electronegativity
- Page ID
- 1496
Electronegativity is a mensurate of the trend of an atom to attract a bonding pair of electrons. The Pauling scale is the most unremarkably used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to cesium and francium which are the to the lowest degree electronegative at 0.seven.
What if 2 atoms of equal electronegativity bond together?
Consider a bond between ii atoms, A and B. If the atoms are equally electronegative, both accept the same tendency to attract the bonding pair of electrons, and so it will be establish on average half way between the two atoms:
To get a bail like this, A and B would usually have to be the aforementioned atom. You lot will find this sort of bond in, for example, H2 or Cl2 molecules. Note: It'south of import to realize that this is an average picture. The electrons are actually in a molecular orbital, and are moving around all the time within that orbital. This sort of bond could be thought of every bit beingness a "pure" covalent bond - where the electrons are shared evenly between the 2 atoms.
What if B is slightly more than electronegative than A?
B will attract the electron pair rather more than A does.
That means that the B end of the bail has more than its fair share of electron density and so becomes slightly negative. At the aforementioned fourth dimension, the A end (rather short of electrons) becomes slightly positive. In the diagram, "\(\delta\)" (read as "delta") ways "slightly" - and then \(\delta+\) means "slightly positive".
A polar bail is a covalent bail in which there is a separation of charge between one end and the other - in other words in which one end is slightly positive and the other slightly negative. Examples include most covalent bonds. The hydrogen-chlorine bail in HCl or the hydrogen-oxygen bonds in water are typical.
If B is a lot more electronegative than A, and so the electron pair is dragged correct over to B'southward end of the bond. To all intents and purposes, A has lost control of its electron, and B has complete control over both electrons. Ions have been formed. The bond is then an ionic bond rather than a covalent bond.
A "spectrum" of bonds
The implication of all this is that there is no clear-cut division betwixt covalent and ionic bonds. In a pure covalent bail, the electrons are held on average exactly half style between the atoms. In a polar bond, the electrons have been dragged slightly towards ane cease. How far does this dragging have to go before the bond counts equally ionic? There is no real answer to that. Sodium chloride is typically considered an ionic solid, but even here the sodium has not completely lost command of its electron. Considering of the properties of sodium chloride, however, we tend to count information technology every bit if information technology were purely ionic. Lithium iodide, on the other paw, would be described as being "ionic with some covalent grapheme". In this case, the pair of electrons has not moved entirely over to the iodine end of the bond. Lithium iodide, for example, dissolves in organic solvents like ethanol - non something which ionic substances ordinarily practise.
Summary
- No electronegativity difference between 2 atoms leads to a pure non-polar covalent bond.
- A small electronegativity difference leads to a polar covalent bond.
- A big electronegativity difference leads to an ionic bond.
Instance 1: Polar Bonds vs. Polar Molecules
In a elementary diatomic molecule similar HCl, if the bail is polar, then the whole molecule is polar. What nigh more than complicated molecules?
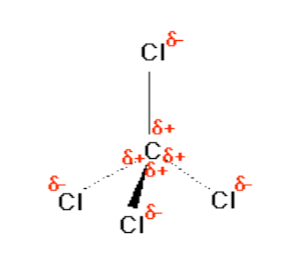

Consider CCl4, (left panel in figure above), which as a molecule is non polar - in the sense that it doesn't take an stop (or a side) which is slightly negative and one which is slightly positive. The whole of the outside of the molecule is somewhat negative, but in that location is no overall separation of charge from top to bottom, or from left to correct.
In contrast, CHCl3 is a polar molecule (correct panel in figure to a higher place). The hydrogen at the top of the molecule is less electronegative than carbon and so is slightly positive. This means that the molecule at present has a slightly positive "top" and a slightly negative "bottom", and and then is overall a polar molecule.
A polar molecule volition need to be "lop-sided" in some way.
Patterns of electronegativity in the Periodic Tabular array
The distance of the electrons from the nucleus remains relatively constant in a periodic table row, but not in a periodic tabular array column. The force between two charges is given by Coulomb's constabulary.
\[ F=g\dfrac{Q_1Q_2}{r^two} \]
In this expression, Q represents a charge, k represents a constant and r is the distance between the charges. When r = 2, so r2= four. When r = three, then r2 = ix. When r = 4, so r2 = 16. It is readily seen from these numbers that, as the altitude betwixt the charges increases, the force decreases very speedily. This is called a quadratic modify.
The effect of this alter is that electronegativity increases from bottom to top in a cavalcade in the periodic table even though there are more than protons in the elements at the bottom of the column. Elements at the summit of a column accept greater electronegativities than elements at the bottom of a given cavalcade.
The overall tendency for electronegativity in the periodic table is diagonal from the lower left corner to the upper right corner. Since the electronegativity of some of the important elements cannot be determined by these trends (they prevarication in the wrong diagonal), we accept to memorize the following guild of electronegativity for some of these common elements.
F > O > Cl > North > Br > I > S > C > H > metals
The most electronegative element is fluorine. If you remember that fact, everything becomes like shooting fish in a barrel, because electronegativity must e'er increase towards fluorine in the Periodic Table.
Note: This simplification ignores the noble gases. Historically this is because they were believed non to form bonds - and if they practice non course bonds, they cannot have an electronegativity value. Fifty-fifty now that we know that some of them practise course bonds, information sources still do not quote electronegativity values for them.
Trends in electronegativity across a period
The positively charged protons in the nucleus attract the negatively charged electrons. As the number of protons in the nucleus increases, the electronegativity or attraction volition increase. Therefore electronegativity increases from left to right in a row in the periodic table. This consequence just holds true for a row in the periodic table because the attraction betwixt charges falls off rapidly with distance. The chart shows electronegativities from sodium to chlorine (ignoring argon since information technology does not does not form bonds).
Trends in electronegativity downwards a group
As you go down a group, electronegativity decreases. (If it increases up to fluorine, it must subtract equally y'all become downwards.) The nautical chart shows the patterns of electronegativity in Groups 1 and 7.
Explaining the patterns in electronegativity
The attraction that a bonding pair of electrons feels for a particular nucleus depends on:
- the number of protons in the nucleus;
- the distance from the nucleus;
- the corporeality of screening by inner electrons.
Why does electronegativity increment across a menses?
Consider sodium at the beginning of period 3 and chlorine at the finish (ignoring the noble gas, argon). Think of sodium chloride as if it were covalently bonded.
Both sodium and chlorine have their bonding electrons in the three-level. The electron pair is screened from both nuclei past the 1s, 2s and 2p electrons, but the chlorine nucleus has 6 more protons in information technology. It is no wonder the electron pair gets dragged so far towards the chlorine that ions are formed. Electronegativity increases across a period considering the number of charges on the nucleus increases. That attracts the bonding pair of electrons more strongly.
Why does electronegativity fall every bit you go downwardly a group?
Every bit you lot go downwardly a group, electronegativity decreases because the bonding pair of electrons is increasingly distant from the attraction of the nucleus. Consider the hydrogen fluoride and hydrogen chloride molecules:
The bonding pair is shielded from the fluorine's nucleus only by the 1s2 electrons. In the chlorine case it is shielded past all the 1s22s22pvi electrons. In each example at that place is a cyberspace pull from the heart of the fluorine or chlorine of +7. But fluorine has the bonding pair in the two-level rather than the 3-level as it is in chlorine. If it is closer to the nucleus, the attraction is greater.
Diagonal relationships in the Periodic Tabular array
At the beginning of periods 2 and 3 of the Periodic Table, there are several cases where an element at the top of one group has some similarities with an element in the next group. Three examples are shown in the diagram below. Notice that the similarities occur in elements which are diagonal to each other - not side-by-side.
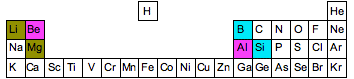
For example, boron is a non-metal with some properties rather like silicon. Unlike the rest of Group ii, glucinium has some properties resembling aluminum. And lithium has some backdrop which differ from the other elements in Group i, and in some means resembles magnesium. At that place is said to be a diagonal relationship between these elements. In that location are several reasons for this, but each depends on the fashion atomic backdrop like electronegativity vary effectually the Periodic Table. And then we will take a quick look at this with regard to electronegativity - which is probably the simplest to explain.
Explaining the diagonal relationship with regard to electronegativity
Electronegativity increases across the Periodic Tabular array. So, for example, the electronegativities of beryllium and boron are:
Electronegativity falls as yous become down the Periodic Table. So, for example, the electronegativities of boron and aluminum are:
And then, comparing Be and Al, you find the values are (by hazard) exactly the same. The increase from Grouping 2 to Group 3 is commencement past the fall equally yous go down Grouping 3 from boron to aluminum. Something similar happens from lithium (1.0) to magnesium (1.2), and from boron (2.0) to silicon (1.8). In these cases, the electronegativities are not exactly the aforementioned, only are very close.
Like electronegativities between the members of these diagonal pairs means that they are likely to form similar types of bonds, and that volition touch their chemical science. You may well come across examples of this later on in your course.
Contributors and Attributions
-
Jim Clark (Chemguide.co.uk)
- Prof. Richard Bank, Boise State University, Emeritus,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electronegativity
Posted by: franksconot1980.blogspot.com


0 Response to "How To Find Electronegativity With Periodic Table"
Post a Comment